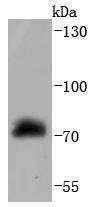
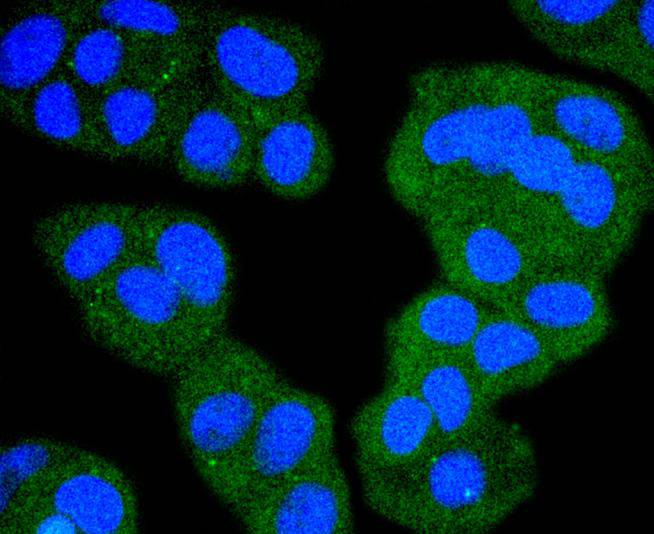
Several serine/threonine protein kinases have been implicated as intermediates in signal transduction pathways. These include ERK/MAP kinases, ribosomal S6 kinase (Rsk)and Raf-1. Raf-1 is a cytoplasmic protein with intrinsic serine/threonine activity. It is broadly expressed in nearly all cell lines tested to date and is the cellular homolog of v-Raf, the product of the transforming gene of the 3611 strain of murine sarcoma virus. The unregulated kinase activity of the v-Raf protein has been associated with transformation and mitogenesis while the activity of Raf-1 is normally suppressed by a regulatory N-terminal domain. Raf-1 is activated in response to activation of a variety of tyrosine kinase receptors as well as in response to pp60v-Src expression. There is accumulating evidence that Ras p21 may play a role in activation of Raf-1 and may play the role of the messenger from membrane tyrosine kinases to Raf-1.