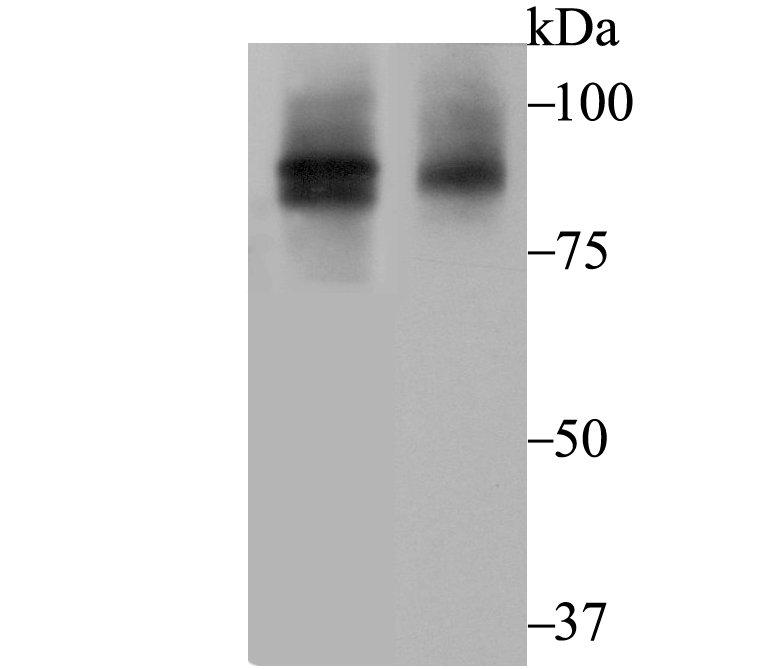
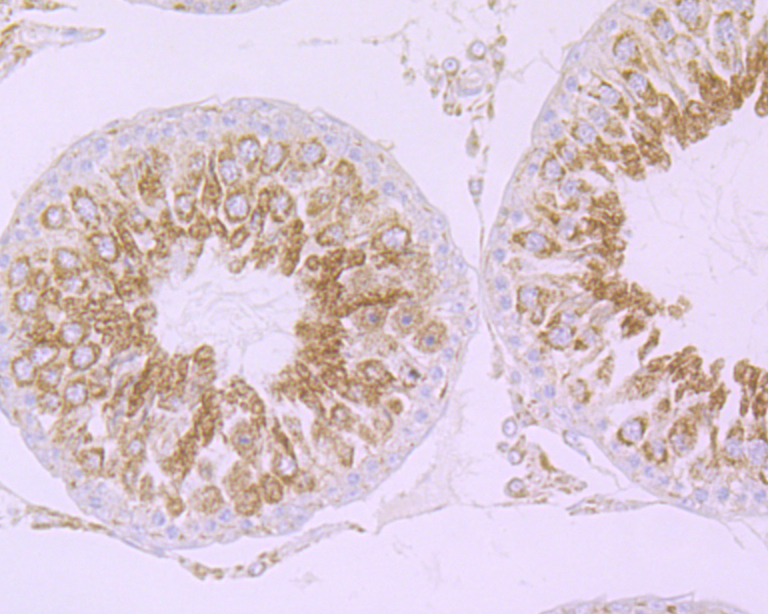
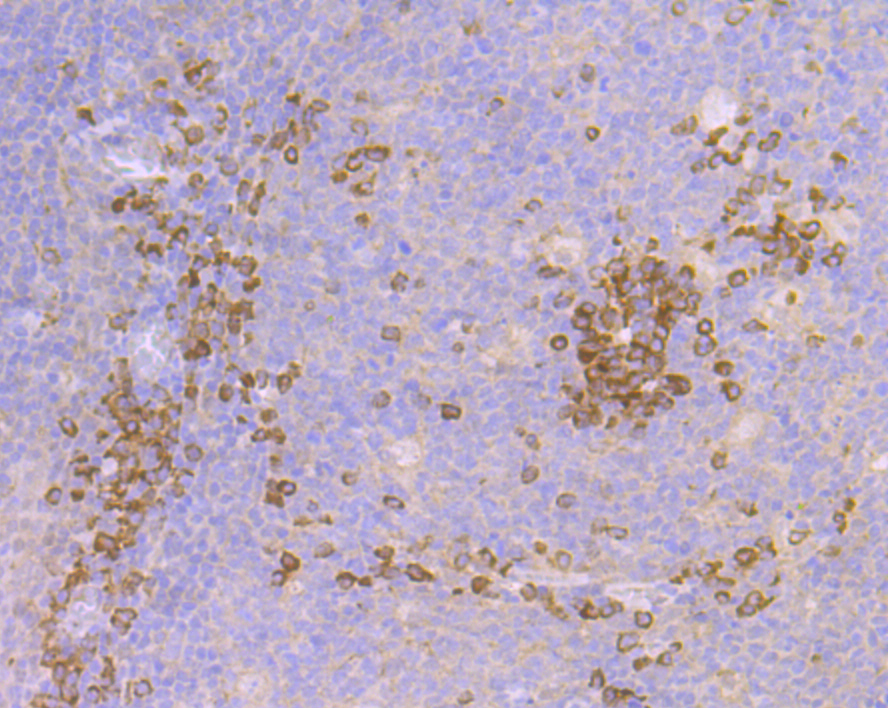
Cullin proteins comprise a distinct family of mediators that participate in the selective targeting of proteins for ubiquitin (Ub)-mediated proteolysis. CUL-1, which is the mammalian homolog of yeast Cdc53, is an integral component of the E3 ubiquitin ligase complex designated SCF. The SCF (Skp1/CUL-1/F-box protein complex) consists of Skp1 associating with both CUL-1 and an F-box protein, such as Skp2, which determines the substrate specificity of the complex. CUL-1-mediated ubiquitination results in the degradation of cell cycle proteins cyclin D, p21 and cyclin E. Another cullin, CUL-3, facilitates the degradation of cyclin E independent of SCF activity, while CUL-2 associates with the tumor suppressing protein VHL and elongin B to form VBC complexes, which structurally resemble the SCF ligase. Proteolysis also occurs by way of CUL-4 associating with Nedd-8, a ubiquitin-like protein, where it too functions as an active component of a multifunctional E3 complex.